Green hydrogen produced by water splitting using renewable energy is the most promising energy carrier of the low-carbon economy. However, the geographic mismatch between renewables distribution and freshwater availability poses a significant challenge to its production.
Here, we demonstrate a method of direct hydrogen production from the air, namely, in situ capture of freshwater from the atmosphere using hygroscopic electrolyte and electrolysis powered by solar or wind with a current density up to 574 mA cm−2. A prototype of such has been established and operated for 12 consecutive days with a stable performance at a Faradaic efficiency around 95%.
This so-called direct air electrolysis (DAE) module can work under a bone-dry environment with a relative humidity of 4%, overcoming water supply issues and producing green hydrogen sustainably with minimal impact to the environment. The DAE modules can be easily scaled to provide hydrogen to remote, (semi-) arid, and scattered areas.
Design of the Direct Air Electrolysis (DAE) module for hydrogen production
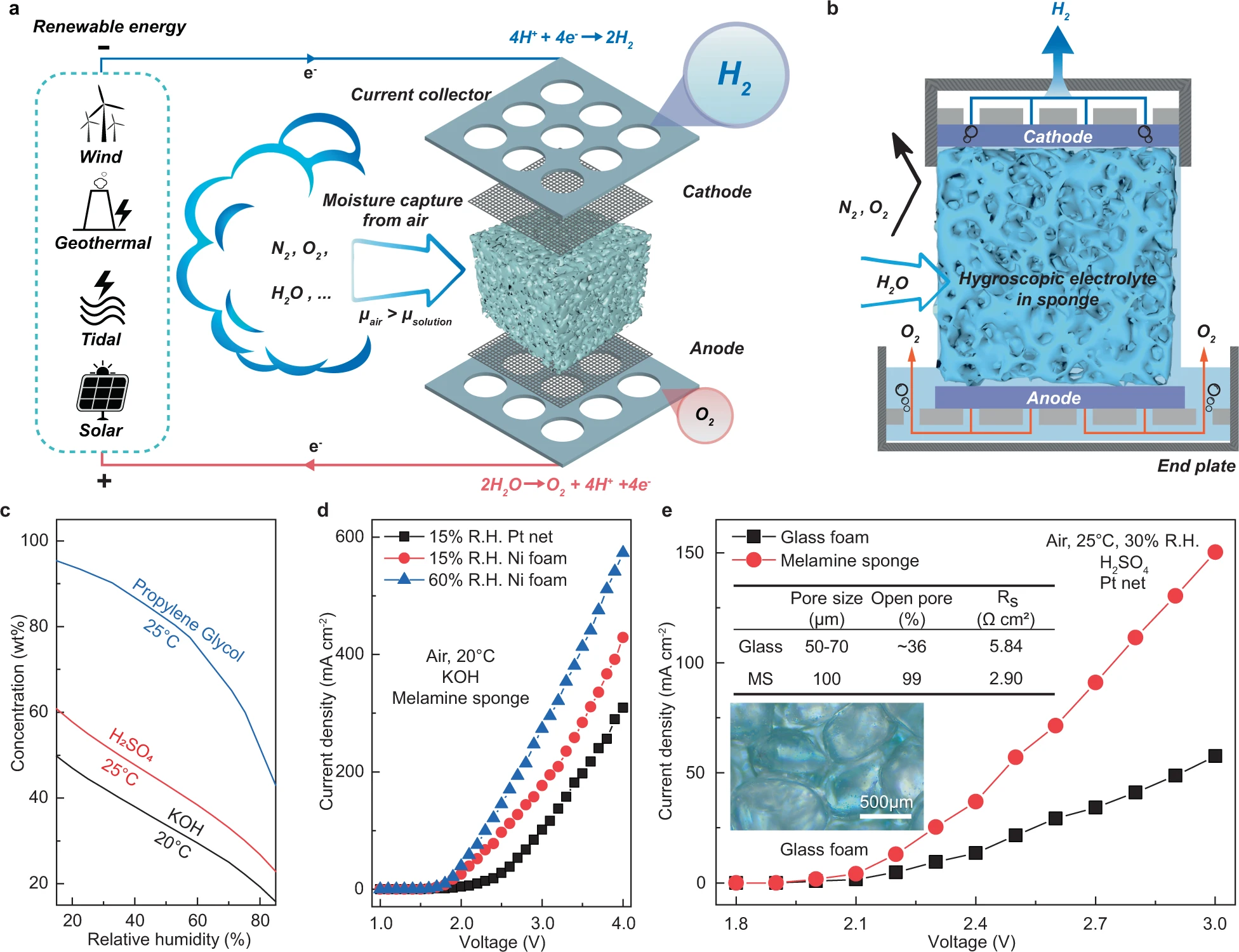
Hydrogen production from the air was realized through our DAE module. this module consists of a water harvesting unit in the middle and electrodes on both sides paired with gas collectors. The module is integrated with a power supply, for example, a solar panel, a wind turbine, and any other renewable generators. Importantly, the water harvesting unit also serves as the reservoir to hold the electrolyte. Porous medium such as melamine sponge, sintered glass foam is soaked with deliquescent ionic substance to absorb moisture from the air via the exposed surfaces.
The captured water in the liquid phase is transferred to the surfaces of the electrodes via diffusion and subsequently split into hydrogen and oxygen in situ which are collected separately as a pure gas, since both electrodes are isolated from air. The reservoir between the endplate and the porous foam works as an air barrier and a buffer for the volume of the ionic solution at excessive fluctuation of the air humidity. This reservoir avoids the overflow of the electrolyte from the DAE module or the dry-up of the wetted foam. When glass foam is chosen as the porous media, quartz wool is tightly packed in between the foam and the electrodes to ensure the connectivity of the aqueous phase. The porous media also ensure the free movement of the electrolyte in the capillary of the foam. The foam filled with ionic solutions forms a physical barrier that effectively isolates hydrogen, oxygen, and air from any mixing.
This is what is known as a direct electrolysis module, in which spongy materials similar to silica gel, as is known from dehumidifier packages, serve as electrodes. These materials draw water from the surrounding air and channel it into a chamber where the electrolysis process takes place using a wind turbine. The energy of a solar module should be sufficient to operate the system. The resulting hydrogen is collected, and the remaining oxygen is released into the environment.
Literature:
Guo, J., Zhang, Y., Zavabeti, A. et al. Hydrogen production from the air. Nat Commun 13, 5046 (2022).
https://doi.org/10.1038/s41467-022-32652-y
This article is licensed under a Creative Commons Attribution 4.0 International License.
Source: Nature Communications, September 6, 2022
https://www.nature.com/articles/s41467-022-32652-y#citeas

